Fire and gas characterization for li-ion cell and battery fires
Lithium-ion batteries have the highest energy density of all rechargeable battery chemistries. Batteries of this chemistry have a very good cycle life, rate capability, lack memory effect and are lightweight and occupy less volume compared to other rechargeable battery systems. The commercialization of Li-ion batteries started in the mid-1990s and picked up speed very quickly. Today, Li-ion batteries are used in almost all consumer portable device applications, and their use has been extended to very large systems as those used in aerospace, EVs and stationary grid energy storage. They have also been used in large applications by the US Navy and are being incorporated into commercial marine applications.
The transition from the use of Li-ion in portable applications to these significantly large ones brings about a lot of challenges. Accidental release of energy packed in Li-ion cells may be prone to fire and smoke, eventually leading to explosions. Even during their initial use in the low power portable applications, recalls from the cell and battery manufacturers were not uncommon. So, the use of this battery chemistry in kilowatt-hour (KWh) to megawatt-hour (MWh) sizes, in the various sectors that they have been introduced into, does bring about new concerns for the regulators and safety officers. For instance, the introduction of Li-ion batteries in automobiles have caused mixed reactions from consumers. Consumers interested in a greener environment feel the obligation to procure and use some form of an electrified vehicle, while others are concerned about the safety of the more recently introduced technology that powers the vehicles.
EVs can be of different forms from hybrid and plug-in hybrids to pure EVs. In the late 1990s and early 2000, hybrid EVs were mainly powered by nickel-metal hydride batteries. With the increase in the types of electrified vehicles and the proliferation of the Li-ion battery chemistry, more vehicles were fitted with Li-ion batteries. A study by the National Fire Protection Association (NFPA) on vehicle fire incidents showed that in the US, between the period of 2003 to 2007, an average of 287,000 vehicle fires had been reported per year and the vehicle fires constituted 17 percent of all reported fires in the US.
In August 2012, the National Highway Traffic Safety Administration (NHTSA) carried out a study with General Motors (GM) and published a report that indicated that the overall consequences of Li-ion battery vehicle fires are expected to be less than those for the gasoline or diesel vehicular fires, due to the much smaller amounts of flammable solvent released and burning in a catastrophic failure situation. This was also based on the statement that the ignition of flammable electrolytic solvents used in Li-ion battery systems are anticipated to be somewhat comparable to or perhaps slightly less than those for gasoline or diesel vehicle fuels.
In a more recent safety report by the National Transportation Safety Board (NTSB), the following conclusion was stated – "Fires in EVs powered by high-voltage Li-ion batteries pose the risk of electric shock to emergency responders from exposure to the high-voltage components of a damaged Li-ion battery. A further risk is that damaged cells in the battery can experience thermal runaway, which can lead to hazards such as battery reignition/fire."
There have been several Li-ion battery vehicle fire incidents reported (Figure 1) and these include pure EVs, hybrid, and plug-in hybrid vehicles, and some electric ships. Fires related to stationary energy storage have also increased in number with as many as 28 energy storage system (ESS) fires reported from South Korea in 2018. The main causes cited ranged from human error in system integration to the lack of test and analysis of the ESS safety control systems. A major issue found with these large ESS is the loss of data when a catastrophic event occurs, which prevents important knowledge from being transferred to the first responders.
Extensive work has been carried out by private and government organizations in characterizing the hazard causes associated with Li-ion cells and batteries in various applications and environments. However, the area of characterizing Li-ion battery fires, which is typically the result of uncontrolled hazard causes, has not been explored much. Larsson et al have carried out significant work on small Li-ion cells and have been able to identify and characterize the components of the fire and smoke. Yan et al discuss studies carried out on 18650 Li-ion cells and a laptop battery design to understand the failure and propagation of thermal runaway, but the study does not include characterization of the components released during a fire or thermal runaway.
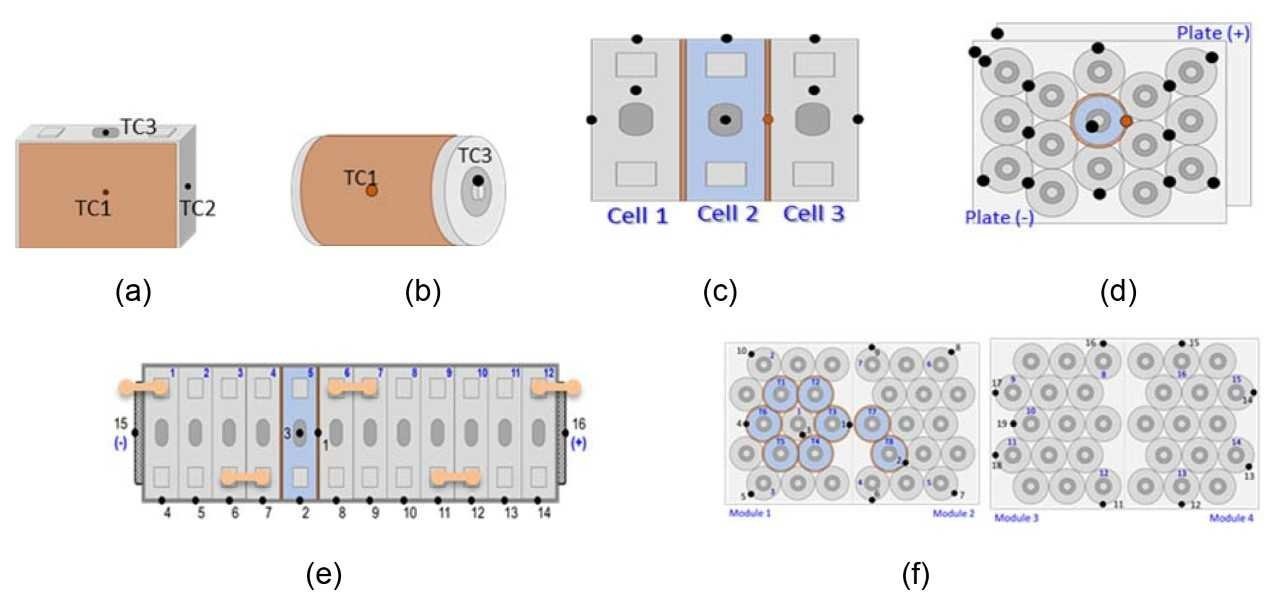
The study carried out at Underwriters Laboratories (UL) included the thermal runaway tests on single cells, a bank of cells, and a module or battery. The module or battery design had a configuration where the banks were connected in series. Two different battery chemistries were studied, namely, Li-ion cells with NMC (nickel, manganese, and cobalt) cathode and with LFP (lithium iron phosphate - LiFePO4) cathode. The Li-ion cells with the NMC cathode were of 25 Ah capacity and a nominal voltage of 3.8 V; the bank composed of 3 cells in parallel (3P) had a capacity of 75 Ah and a nominal voltage of 3.8 V, and the module was composed of four banks in series (3P4S) with a capacity of 75 Ah and a nominal voltage of 15.2 V.
The Li-ion cells with the LFP cathode were of 5.5 Ah capacity and a nominal voltage of 3.3 V; the bank composed of 15 cells in parallel had a capacity of 82.5 Ah and a nominal voltage of 3.3 V; and the battery composed of four banks in series (15P4S) had the capacity of 82.5 Ah and a nominal voltage of 13.2 V. Figure 2 has drawings of the cells, banks, and module/battery designs used in this test program. Thermal runaway was initiated by the use of a heating tape wrapped on the side of the cell or around the cells for the single-cell test. In the bank and module/battery level tests, more than one heating tape was needed to initiate thermal runaway propagation and achieve worst-case fire and smoke. All test articles were tested in a fully charged condition in order to study the worst-case scenarios.
The results of the tests showed that combinations of smoke and fire were observed in all cases. With the LFP test articles, more smoke was observed than fire. The composition of the gases emitted from the test articles provided significant insights into the potential hazards resulting from battery failures.
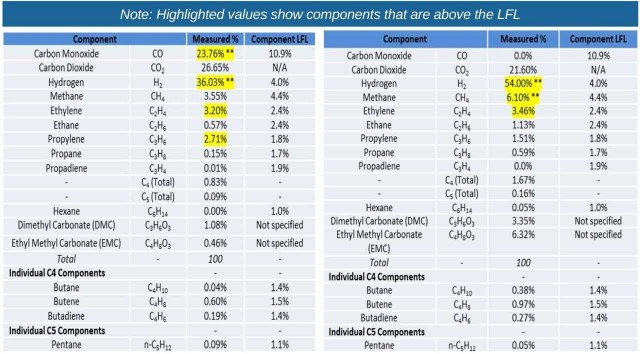
Table 1 shows the compositions of the various gaseous components and their lower flammability limits (LFL), for the NMC and LFP cells studied. For the single 75 Ah NMC cell (95 Wh), about 41L of gas was released and for the single 5.5 Ah LFP cell (18 Wh), about 3L of gas was released. For the NMC module (1.1 kWh) the total hydrocarbon volume that was flammable was 165L and the volume of hydrogen gas was 83L. For the LFP battery (1.1 kWh), the total hydrocarbons released during thermal runaway (no fire was observed with this battery) was 357L and the volume of hydrogen gas was 196L. The table shows that the volume of some of the combustible gases was several times greater than the LFL.
A challenge with gas composition analysis has been the detection of hydrogen fluoride (HF) gas. It is well known that this is a component of all Li-ion cells manufactured today since the electrolyte salt and the binder used in making the electrodes contain fluorine. However, due to the nature of the gas, which condenses at ambient temperatures and has high reactivity, it has been difficult to detect and measure in this study.
Larsson et al have measured HF gas in their single-cell tests using an open test environment. Besides HF, another toxic gas that has been reported in battery fires is hydrogen cyanide (HCN). The presence of HCN above the toxicity limit was reported from the gas analysis carried out in the Arizona battery ESS. Li-ion cells do not produce HCN so this gas may be a byproduct of the burning of other components such as plastics used in the manufacturing of the battery ESS.
In summary, the studies showed that toxic and flammable gases are released from Li-ion cells when they experience thermal runaway and fire. The level of toxicity and the combustible nature of the gases depends a lot on the volume in which they are released into and trapped. Hence, one should take the free volume of the location they are installed in to determine the toxicity and flammability of the released gases. The nature of the gaseous components depends significantly on the chemistry of the Li-ion cell as well as the composition of the electrolyte solvents and salts. A good understanding of the gas composition is imperative for battery installations such as stationary grid ESS, or when vehicle battery charging is carried out in a poorly ventilated area, to provide the required information to first responders and firefighters.
More information about these studies can be obtained by contacting
The above article is authored by a team of researchers at the Underwriters Laboratories - Judith Jeevarajan Ph.D. (Research Director), Daniel Juarez Robles Ph.D. (Research Scientist), Tapesh Joshi Ph. D. (Research Scientist), and Kanarindhana Kathirvel (Program Manager).
The authors acknowledge Dr. Pravin Gandhi, Alex Klieger, and their UL Fire Research Team for assistance in carrying out the tests; and Saad Azam (graduate student at Dalhousie University) for his contributions to the test program. The research work carried out has been done with Underwriters Laboratories' internal research funds.